Toxicological Assessment of Medicinal Herbs to Identify Adverse Effects on Eukaryotic Cells
Abstract:
Bibliography/Citations:
Adan, Aysun et al. “Cell Proliferation and Cytotoxicity Assays.” Current pharmaceutical biotechnology vol. 17,14 (2016): 1213-1221. doi:10.2174/1389201017666160808160513
Avigan, Mark I et al. “Scientific and Regulatory Perspectives in Herbal and Dietary Supplement Associated Hepatotoxicity in the United States.” International journal of molecular sciences vol. 17,3 331. 3 Mar. 2016, doi:10.3390/ijms17030331
Botstein, D et al. “Yeast as a model organism.” Science (New York, N.Y.) vol. 277,5330 (1997): 1259-60. doi:10.1126/science.277.5330.1259
Buggapati, Lahari. “Herbs in Dentistry.” International Journal of Pharmaceutical Science Invention, Government Dental College, Vijayawada, Andhra Pradesh, India, Oct. 2016, www.ijpsi.org/Papers/Vol5(6)/C050607012.pdf.
Cruz Martínez, Cindy et al. “Use of traditional herbal medicine as an alternative in dental treatment in Mexican dentistry: a review.” Pharmaceutical biology vol. 55,1 (2017): 1992-1998. doi:10.1080/13880209.2017.1347188
Eisenberg, David M., et al. “Unconventional Medicine in the United States -- Prevalence, Costs, and Patterns of Use: NEJM.” New England Journal of Medicine, 14 Oct. 1993, www.nejm.org/doi/full/10.1056/NEJM199301283280406.
Ekor, Martins. “The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety.” Frontiers in pharmacology vol. 4 177. 10 Jan. 2014, doi:10.3389/fphar.2013.00177
Karimi, Ali et al. “Herbal versus synthetic drugs; beliefs and facts.” Journal of nephropharmacology vol. 4,1 27-30. 1 Jan. 2015
Kumar, Gunjan et al. “Emerging trends of herbal care in dentistry.” Journal of clinical and diagnostic research: JCDR vol. 7,8 (2013): 1827-9. DOI:10.7860/JCDR/2013/6339.3282
Liu, Qing et al. “Antibacterial and Antifungal Activities of Spices.” International journal of molecular sciences vol. 18,6 1283. 16 Jun. 2017, doi:10.3390/ijms18061283
Pelkonen, Olavi et al. “Why is Research on Herbal Medicinal Products Important and How Can We Improve Its Quality?.” Journal of traditional and complementary medicine vol. 4,1 (2014): 1-7. doi:10.4103/2225-4110.124323
Riss, Terry L. “Cell Viability Assays.” Assay Guidance Manual [Internet]., U.S. National Library of Medicine, 1 July 2016, www.ncbi.nlm.nih.gov/books/NBK144065/.
Terrie, Yvette C. “Using Herbal Supplements Safely.” Https://Www.pharmacytimes.com, 19 Mar. 2012, www.pharmacytimes.com/view/-using-herbal-supplements-safely.
Yang, Bo, et al. “Nephrotoxicity and Chinese Herbal Medicine.” American Society of Nephrology, American Society of Nephrology, 8 Oct. 2018, cjasn.asnjournals.org/content/13/10/1605.
“Herbal Medicine Market Size: Share: Global Industry Report, 2026.” Polaris Market Research: Global Market Research Reports and Consulting, Polaris Market Research, Apr. 2020, www.polarismarketresearch.com/industry-analysis/herbal-medicine-market.
“WHO Global Report on Traditional and Complementary Medicine 2019.” World Health Organization, World Health Organization, Jan. 2019, apps.who.int/iris/handle/10665/312342.
Yesh, Sharma. “Herbal Medicines - A Natural Cure In Dentistry.” International Journal of Research and Review, Department of Conservative Dentistry and Endodontics, Maharaja Ganga Singh Dental College, Sriganganagar, Rajasthan, June 2018, www.ijrrjournal.com/IJRR_Vol.5_Issue.6_June2018/IJRR0019.pdf.
Additional Project Information
Research Plan:
The maceration or cold extraction method will be used to prepare herbal extracts. Herbs will be washed in clear water and dried until they will be adequately dry to be ground. Dried leaves will be powdered separately in an electric grinder until a homogenous powder is obtained. Herbal powder purchased from vendors will also be further ground in an electric grinder. A total of 100 g of the finely powdered herb will be macerated with 100% ethanol for 3 days with frequent agitation. This process is intended to soften and break the plant’s cell wall to release the soluble phytochemicals. This alcoholic decoction will be subjected to filtration with Whatman #1 filter paper to obtain a clear filtrate. This filtrate thus obtained will be reduced at a low temperature of less than 60°F to obtain a solid residue of herbal extract.
For this research, baker’s yeast (Saccharomyces Cerevisiae) will be used to grow free-floating yeast cell culture in the culture medium (suspension culture). The yeast–peptone–dextrose (YPD) culture medium, composed of 2% glucose, 1% yeast extract and 2% bacto peptone, will be kept incubated at an optimum temperature of 30°C for 72 hours to grow cells exponentially. Similarly, cell culture will be prepared for E. coli k-12 strains using LB (Luria Broth) broth growth medium consisting of 10g/L tryptone, 10g/L NaCl and 5g/L yeast extract. After growing cells in the starting flask reach the exponential growth phase, contents will be mixed well to have a uniform concentration of cells in the flask and for all the wells to have the same starting number of cells. Cell cultures in 50μl volume (culture medium) will be carefully pipetted into wells ( washing or cell harvesting not required with this cell assay) and will be kept at a concentration of 105 cells/well in a 96-well microplate. The yeast cells will be treated with different concentrations of 50μl volume of test plant extracts ( 50, 150, 250 mg/ml) against the positive control (0.25% fluconazole) and the negative control which contained only the medium (distilled water) and incubated for 48 hours at 37°C. The E. coli k-12 cells will be treated with different concentrations of 50μl volume of test plant extracts ( 50, 150, 250 mg/ml) against the positive control (0.2% chlorhexidine) and the negative control which contained only the medium(distilled water) and incubated for 48 hours at 37°C. 20μl of the combined MTS/PMS solution will be pipetted into each well of the 96 well assay plate containing 100μl of cells in culture medium. Plate will be incubated for 1–4 hours at 37°C in a humidified, 5% CO2 atmosphere. Absorbance will be recorded at 490 nm using an ELISA plate reader
Questions and Answers
1. What was the major objective of your project and what was your plan to achieve it?
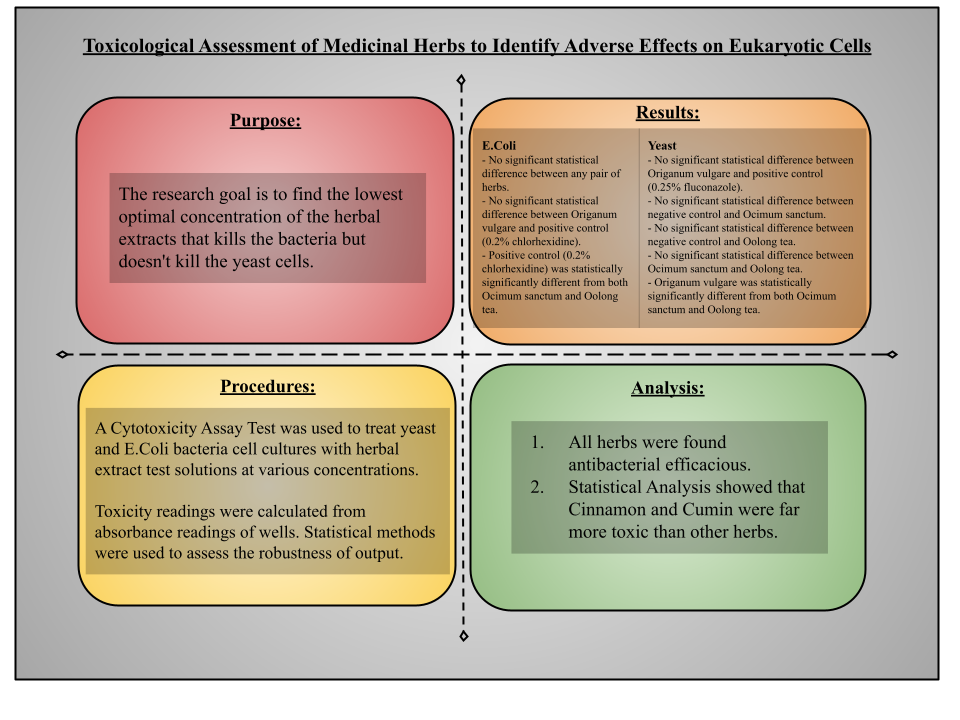
The specific research goal of this project was to find toxicity of medicinal herbs (Ocimum sanctum, oolong tea, and Origanum vulgare), having known efficacy against bacterial microorganisms, to verify the potential pharmacological effects on eukaryotic cells. In simple words, the research goal is to find the lowest concentration of the herbal extracts that kills the bacteria but doesn't kill the yeast cells.
a. Was that goal the result of any specific situation, experience, or problem you encountered?
Two years ago, my grandma was passing out occasionally, but her biomarkers showed no sign of concern. So, in my junior year, when it was time to decide on an independent research project, I didn’t have to think twice. I set out to test three herbs my grandma was using in her cooking for a toxicological assessment. The damage had already been done. She was using the herbs way beyond safe dosage as per my analysis of dead cells in the cytotoxicity assays research
b. Were you trying to solve a problem, answer a question, or test a hypothesis?
Yes, I was trying to test a hypothesis.
2. What were the major tasks you had to perform in order to complete your project?
a. For teams, describe what each member worked on.
Literature review
Grant proposal
Design the experiment
Sourcing herbs, chemicals, and equipment.
Herbal extraction
Cell culture preparation
Incubation of cell culture with herbal extract treatments
Absorbance readings with a microplate reader
Data Calculations and Statistical Analysis
Presentation preparation
3. What is new or novel about your project?
a. Is there some aspect of your project's objective, or how you achieved it that you haven't done before?
b. Is your project's objective, or the way you implemented it, different from anything you have seen?
c. If you believe your work to be unique in some way, what research have you done to confirm that it is?
Despite the large number of people using alternative medicine treatments including medicinal herbs, relatively little scientific data are available to demonstrate convincingly whether or not a particular treatment is safe, beneficial, helpful, or leads to a positive outcome (Obidike, 2013). Depending on toxicological assessment of medicinal herbs, It would be far easier and practical to introduce medicinal herbs for the treatment of short term infections or preventive care than for the longer term cures in medicine where extensive research and in vivo clinical trials are needed before any application of drugs commercially. This research at hand is a focussed effort in the direction of classifying cytotoxicity data of widely used medicinal herbs, having high efficacy against bacterial infections, which can be put to immediate use for further in vivo testing.
Based on results of in vitro experiments, many available studies concluded antibacterial and antifungal efficacy of medicinal herbs for treating various health problems but relatively little scientific data are available to demonstrate convincingly their mechanism of actions, toxicity and resulting side effects on humans, which warrants serious research in this area to generate the required evidence (Cruz, 2017).
This project would significantly help in filling research gaps between traditional knowledge and proven evidence of herbal safety. There is a dearth of studies of highly efficacious medicinal herbs that classified toxicity data which can be immediately used before conducting expensive in vivo clinical trials. There is a significant gap between traditional knowledge and trials investigating medical plants. Additionally, There are gaps in the quality control of herbal medicine products (Pelkonen, 2014).
This research effort is different from some other previous research attempts as it is focussed on some widely used medicinal herbs such as Ocimum sanctum, oolong tea, and Origanum vulgare, which were found to be as effective as commercially available synthetic drugs against most common infections (Buggapati, 2016).
4. What was the most challenging part of completing your project?
a. What problems did you encounter, and how did you overcome them?
This research project was a never ending endeavour. I had designed it so well by spending two months over summer with meticulously including all the details. In a test of my nerves, I had to redesign my experiment multiple times due to shortened lab hours, lack of equipment, and contamination of compounds. I had to figure out an alternative to testing mammalian cells in addition to switching from MTT to MTS based cytotoxity assay due to material safety data concerns in the BSL-1 (Biological Safety Levels) grade high school lab. I sourced used equipment ( ex. Microplate reader) from online scientific equipment vendors.
b. What did you learn from overcoming these problems?
I learned that doing research is hard. It requires tremendous patience and determination. Pressed for time, my colleagues switched to data research. I hadn’t run a single trial by the end of the academic year, but no setback could make me give up. Finally, my endurance paid off, and I was able to get four continuous weeks of lab time to conclude my experiment in the summer.
5. If you were going to do this project again, are there any things you would you do differently the next time?
I used the maceration or cold extraction method to prepare herbal extracts which were very time-consuming. If I was going to do this project again, I would have used the Ultrasound-assisted extraction method due to its several advantages such as low energy consumption, less extraction time, less active compound damage, and high extraction yields.
6. Did working on this project give you any ideas for other projects?
Yes. The widespread use of herbs warrants a study in genotoxicity assessment (using Gene Mutation test in bacteria) to determine any damage to the genetic information within a cell causing mutations that could lead to cancer development.
7. How did COVID-19 affect the completion of your project?
I tried reaching out to various university labs in the area but during the Covid-19 pandemic time, university labs were out of reach as no research lab was entertaining high school students in the area. Due to hybrid learning at high school, research hours were cut drastically and most of us doing research were advised to switch to non-wet lab-based research. At this point, I had to cut short the scope of my experiment by excluding phytochemical analysis from my research. As part of my original research, I really wanted to know the bioactive compounds and their dosage responsible for robust antimicrobial activity against some of the bacteria and fungus microbes I studied during the literature review of existing studies.