Prophages present in Acinetobacter pittii influence bacterial virulence, antibiotic resistance, and genomic structure
Abstract:
Bibliography/Citations:
- Nemec, A., Krizova, L., Maixnerova, M., van der Reijden, T. J. K., Deschaght, P., Passet, V., Vaneechoutte, M., Brisse, S., & Dijkshoorn, L. (2011). Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus–Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU). Research in Microbiology, 162(4), 393–404. https://doi.org/10.1016/j.resmic.2011.02.006
- Chopjitt, P., Putthanachote, N., Ungcharoen, R., Hatrongjit, R., Boueroy, P., Akeda, Y., Tomono, K., Hamada, S., & Kerdsin, A. (2021). Genomic Characterization of Clinical Extensively Drug-Resistant Acinetobacter pittii Isolates. Microorganisms, 9(2), 242. https://doi.org/10.3390/microorganisms9020242
- Chusri, S., Chongsuvivatwong, V., Rivera, J. I., Silpapojakul, K., Singkhamanan, K., McNeil, E., & Doi, Y. (2014). Clinical Outcomes of Hospital-Acquired Infection with Acinetobacter nosocomialis and Acinetobacter pittii. Antimicrobial Agents and Chemotherapy, 58(7), 4172–4179. https://doi.org/10.1128/aac.02992-14
- Murray, C. K., & Hospenthal, D. R. (2008). Acinetobacter Infection in the ICU. Critical Care Clinics, 24(2), 237–248. https://doi.org/10.1016/j.ccc.2007.12.005
- Boo, T. W., Walsh, F., & Crowley, B. (2009). Molecular characterization of carbapenem-resistant Acinetobacter species in an Irish university hospital: predominance of Acinetobacter genomic species 3. Journal of Medical Microbiology, 58(2), 209–216. https://doi.org/10.1099/jmm.0.004911-0
- Cosgaya, C., Ratia, C., Marí-Almirall, M., Rubio, L., Higgins, P. G., Seifert, H., Roca, I., & Vila, J. (2019). In vitro and in vivo Virulence Potential of the Emergent Species of the Acinetobacter baumannii (Ab) Group. Frontiers in Microbiology, 10. https://doi.org/10.3389/fmicb.2019.02429
- Larcher, R., Pantel, A., Arnaud, E., Sotto, A., & Lavigne, J.-P. (2017). First report of cavitary pneumonia due to community-acquired Acinetobacter pittii, study of virulence and overview of pathogenesis and treatment. BMC Infectious Diseases, 17(1). https://doi.org/10.1186/s12879-017-2589-0
- Lee, Y.-C., Huang, Y.-T., Tan, C.-K., Kuo, Y.-W., Liao, C.-H., Lee, P.-I., & Hsueh, P.-R. (2011). Acinetobacter baumannii and Acinetobacter genospecies 13TU and 3 bacteraemia: comparison of clinical features, prognostic factors and outcomes. Journal of Antimicrobial Chemotherapy, 66(8), 1839–1846. https://doi.org/10.1093/jac/dkr200
- Fortier, L.-C., & Sekulovic, O. (2013). Importance of prophages to evolution and virulence of bacterial pathogens. Virulence, 4(5), 354–365. https://doi.org/10.4161/viru.24498
- Ramisetty, B. C., & Sudhakari, P. A. (2019). Bacterial ‘Grounded’ Prophages: Hotspots for Genetic Renovation and Innovation. Frontiers in Genetics, 10. https://doi.org/10.3389/fgene.2019.00065
- Costa, A. R., Monteiro, R., & Azeredo, J. (2018). Genomic analysis of Acinetobacter baumannii prophages reveals remarkable diversity and suggests profound impact on bacterial virulence and fitness. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-33800-5
- Loh, B., Chen, J., Manohar, P., Yu, Y., Hua, X., & Leptihn, S. (2020). A Biological Inventory of Prophages in A. baumannii Genomes Reveal Distinct Distributions in Classes, Length and Genomic Positions. Frontiers in Microbiology. https://doi.org/10.1101/2020.10.26.355222
- NCBI Resource Coordinators (2018). Database resources of the National Center for Biotechnology Information. Nucleic acids research, 46(D1), D8–D13. https://doi.org/10.1093/nar/gkx1095
- Arndt, D., Grant, J. R., Marcu, A., Sajed, T., Pon, A., Liang, Y., & Wishart, D. S. (2016). PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Research, 44(W1). https://doi.org/10.1093/nar/gkw387
- Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. ISBN 978-3-319-24277-4, https://ggplot2.tidyverse.org
- Overbeek, R., Olson, R., Pusch, G. D., Olsen, G. J., Davis, J. J., Disz, T., Edwards, R. A., Gerdes, S., Parrello, B., Shukla, M., Vonstein, V., Wattam, A. R., Xia, F., & Stevens, R. (2013). The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Research, 42(D1). https://doi.org/10.1093/nar/gkt1226
- Liu, B., Zheng, D., Jin, Q., Chen, L., & Yang, J. (2018). VFDB 2019: a comparative pathogenomic platform with an interactive web interface. Nucleic Acids Research, 47(D1). https://doi.org/10.1093/nar/gky1080
- Darling, A. E., Mau, B., & Perna, N. T. (2010). progressiveMauve: Multiple Genome Alignment with Gene Gain, Loss and Rearrangement. PLoS ONE, 5(6). https://doi.org/10.1371/journal.pone.0011147
- Alcock, B. P., Raphenya, A. R., Lau, T. T., Tsang, K. K., Bouchard, M., Edalatmand, A., Huynh, W., Nguyen, A.-L. V., Cheng, A. A., Liu, S., Min, S. Y., Miroshnichenko, A., Tran, H.-K., Werfalli, R. E., Nasir, J. A., Oloni, M., Speicher, D. J., Florescu, A., Singh, B., … McArthur, A. G. (2019). CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Research. https://doi.org/10.1093/nar/gkz935
Additional Project Information
Project files
Research Plan:
Rationale:
Antibiotic resistance and virulence are common among bacteria, posing a global clinical challenge. The bacterial species Acinetobacter pittii—a cause of nosocomial infections—has shown increasing rates of antibiotic resistance, especially in regards to carbapenem antibiotics. A. pittii’s increasing antibiotic resistance and virulence warrants further study.
One mechanism of A. pittii pathogenicity could be through prophages. Prophages are formed by bacteriophages when they integrate their viral genome into bacterial genomes. Prophages often contain virulence factors (proteins that enhance the pathogenicity of their host) and thus increase the survival fitness of bacteria.
Prophages have also been associated with virulence and antibiotic resistance in other Acinetobacter species. In A. baumannii, prophages have been identified that encode a variety of virulence factors ranging from efflux pumps (which remove) toxins to antibiotic-inactivating enzymes. However, while close relatives like A. baumannii have been surveyed for integrated viral sequences, such prophage analysis has yet to be applied to A. pittii. Given the clinical infections resulting from A. pittii, it is imperative that the mechanisms of its pathogenicity be better understood. As a result, by identifying the specific prophages and virulence factors that are most common in A. pittii, this study could provide new directions for A. pittii research and pinpoint existing or future causes of its virulence.
Our results will have clinical implications, helping researchers understand a bacterial responsible for infections in the hospital setting. Moreover, the results provide broader insight into prophage behavior for the Acinetobacter genus, helping researchers better characterize this group of bacteria, which has multiple clinically-significant species.
Research Questions:
1. How many prophages do Acinetobacter pittii strains contain? How do they differ depending on the viral family they are derived from?
2. What types of antibiotic resistance genes and virulence factors are contained within these prophages? To what extent are these genes present with high confidence within the prophage sequences?
3. In what other ways may these prophages influence A. pittii pathogenicity? What effects do they have on genomic structure and diversity?
Procedures:
1. Collect complete Acinetobacter pittii genomes from all available strains in NCBI GenBank.
2. Use the PHAge Search Tool - Enhanced Release (PHASTER) web server to identify potential prophages in each A. pittii strain. Select only the prophages that PHASTER labels as the highest confidence (Intact) to continue with analysis.
3. Characterize the intact prophages. Specifically, run a one-tailed t-Test assuming unequal variances on the prophage length for each viral family represented (p < 0.05).
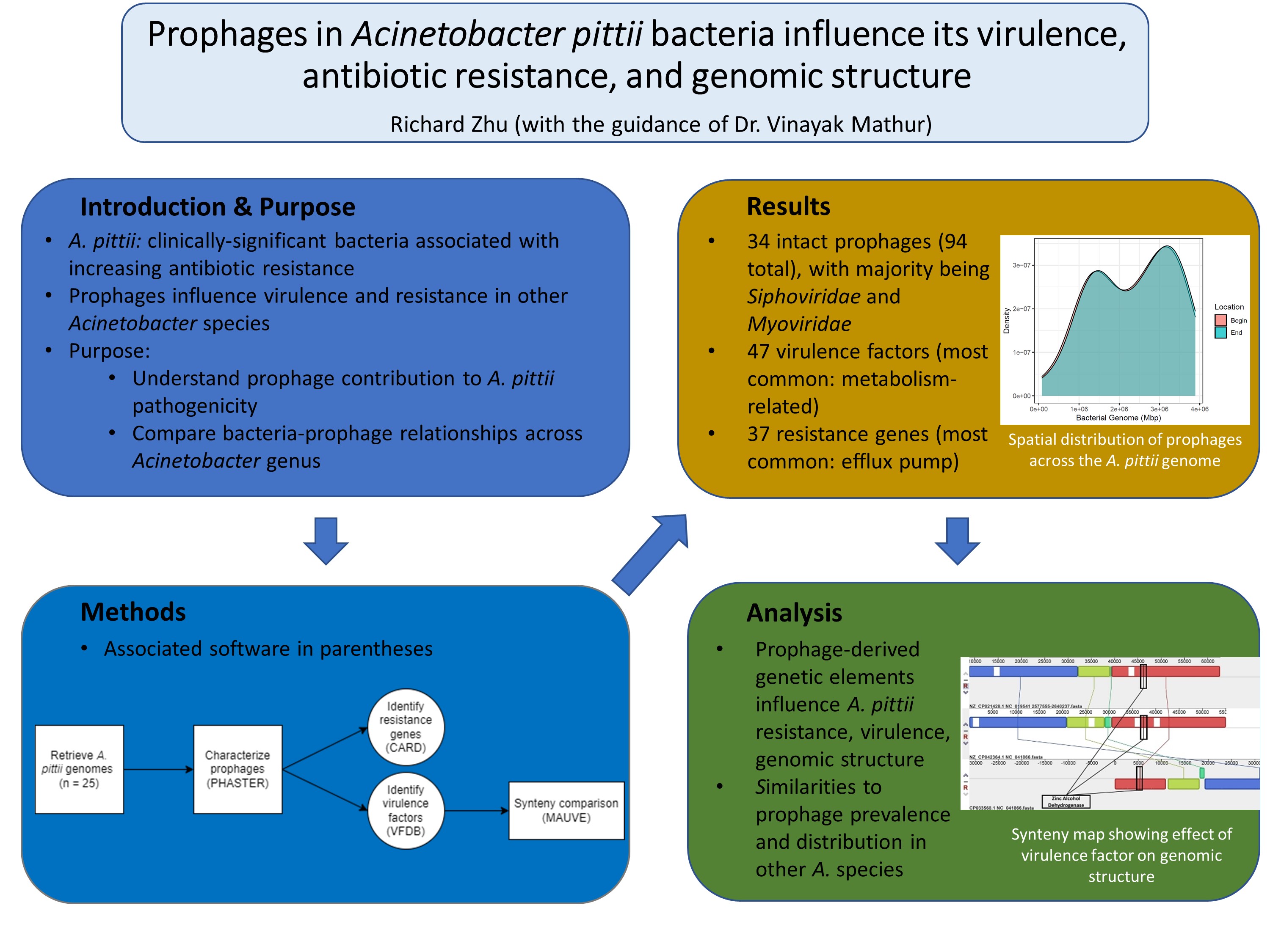
4. Make a density plot of the spatial distribution of intact prophages across the A. pittii chromosome.
5. Annotate all bacterial genomes containing intact prophages. Then, compare the prophage sequences in these genomes to the Virulence Factor DataBase (VFDB) with default parameters to identify virulence factors within the prophage sequences. The Expect value cutoff is set as 1 × 10-20.
6. Categorize prophage virulence factors by function using a review of the scientific literature.
7. To analyze the effect of virulence factors on genomic structure, identify all prophages which share the two most common virulence factors. Then, generate synteny maps in Mauve for these two sets of prophages.
8. Identify antibiotic resistance genes within the prophage sequences by comparing intact prophage sequences against the Comprehensive Antibiotic Resistance Database (CARD). Categorize these prophage resistance genes by function based on their labeling in CARD.
9. Using a phylogenetic tree in MEGA, compare the YMC/09/02/B1251_ABA_BP prophage in A. baumannii and A. pittii to confirm its prevalence in both Acinetobacter species.
Risk and Safety: Not applicable.
Questions and Answers
1. What was the major objective of your project and what was your plan to achieve it?
We were interested in determining whether prophages (viral elements integrated into bacterial genomes) contributed to antibiotic resistance and general virulence in the bacteria Acinetobacter pittii. We planned to employ a bioinformatics approach, using databases and online software (i.e. PHASTER for prophage identification) in order to quickly and reliably characterize prophage elements for all available A. pittii strains.
a. Was that goal the result of any specific situation, experience, or problem you encountered?
After reading a study of virulence-enhancing prophages in Acinetobacter baumannii, I wondered if the related and clinically-significant Acinetobacter pittii could also harbor prophages that enhance the virulence and antibiotic resistance of A. pittii. I then performed an extensive literature search to ensure the project idea was novel. The literature search revealed that many species related to A. pittii had been analyzed for prophages in their genome, such as A. baumannii, A. baylyi, and A. nosocomialis, but A. pittii had yet to be extensively studied. This thus clarified the goals for my study.
b. Were you trying to solve a problem, answer a question, or test a hypothesis?
We were trying to test the hypothesis that Acinetobacter pittii contained prophages that contributed significant amounts of virulence factors and antibiotic resistance, genetic elements that could make the bacteria more pathogenic. We were also trying to answer the question of what these virulence-influencing prophages could be and if they influenced the genome in any way other than conferring pathogenicity-enhancing genes.
2. What were the major tasks you had to perform in order to complete your project?
a. For teams, describe what each member worked on.
I performed the literature search to ascertain that the research idea was novel. I developed the hypotheses, designed the experiment, identified prophages using PHASTER software, characterized the virulence factors and resistance genes in these prophages, generated synteny maps to identify the relationship between virulence factors and genomic structure, performed relevant statistical analysis, and wrote the initial drafts of the paper.
Dr. Mathur helped me draw conclusions from the data I gathered and processed. In particular, he helped me organize the Mauve synteny results and notice genomic rearrangement patterns. He also helped me organize, edit, and revise my final research paper.
3. What is new or novel about your project?
As stated previously, Acinetobacter pittii has yet to be analyzed in terms of virulence-enhancing prophages, so all of our results that relate to Acinetobacter pittii prophages and the virulence-related sequences within them are novel.
a. Is there some aspect of your project's objective, or how you achieved it that you haven't done before?
This project was the first time I fully designed and implemented an experimental procedure. I learned multiple new software and bioinformatics techniques, such as genome annotation and prophage identification.
b. Is your project's objective, or the way you implemented it, different from anything you have seen?
The objective of characterizing prophages specifically in Acinetobacter pittii is novel. Moreover, the previous Acinetobacter prophage studies which I looked at (i.e. for Acinetobacter baumannii) did not analyze the effect of virulence factors on genomic structure.
c. If you believe your work to be unique in some way, what research have you done to confirm that it is?
I performed a comprehensive literature review in PubMed and found no study analyzing the relationship between Acinetobacter pittii and prophages, including by labs that had analyzed prophages in other Acinetobacter species. Phrases that I searched for included “acinetobacter pittii prophage,” “acinetobacter pittii virulence,” and “acinetobacter prophage.”
4. What was the most challenging part of completing your project?
a. What problems did you encounter, and how did you overcome them?
b. What did you learn from overcoming these problems?
The greatest challenge in the project was designing the experimental procedure. Since I did not have access to the resources of a wet lab, I needed to obtain and analyze all of my data using online software applications. To do this, I read many previous studies relating to bioinformatics analysis of prophages, which helped me identify the general types of software and workflow required for this project. This project helped me realize the amount and diversity of biological data available in online databases, which opened my eyes to the potential of bioinformatics research.
5. If you were going to do this project again, are there any things you would you do differently the next time?
The study only analyzed 25 A. pittii strains, which were all the strains available in GenBank at the time of my research. However, I would analyze more strains if we were to re-do the project.
In addition, I would experimentally confirm my prophage identification results with more software in addition to PHASTER to ensure higher confidence in my results.
Our virulence factor and resistance gene analyses were performed only on Intact prophages (as identified by PHASTER); extensions of the project could analyze Incomplete and Questionable prophages identified by PHASTER as well, which may provide insight into prophage sequences disrupted by genetic recombination or generally less intact.
Finally, because this study was exclusively computational, I would be eager to experimentally validate the results with wet lab analysis, which would help to confirm that prophage-derived virulence and antibiotic resistance genetic elements influence A. pittii bacteria.
6. Did working on this project give you any ideas for other projects?
Additional projects I would like to pursue include:
- Characterizing prophages in other Acinetobacter species and comparing results with A. pittii to reveal similarities or differences in these bacteria’s relationships to prophages.
- Using molecular biology techniques to identify the mechanisms by which prophage-derived virulence factor sequences can promote genomic structure rearrangements in their vicinity, and whether this mechanism is shared across multiple types of bacteria.
7. How did COVID-19 affect the completion of your project?
Because of the computational nature of the project, COVID-19 did not significantly affect my access to research resources and tools (and thus did not affect completion), apart from occasional WiFi connection interruptions.